12th June 2023
RSC Schools’ Analyst Competition 2023

On Friday 12 May, 22 intrepid BHASVIC chemists competed in small teams during the Schools’ Analyst Competition 2023. This year’s task was to discover the “mystery saboteur” who – as an unscrupulous competitor - has ransacked the pantry at the County Bake-off and tampered with the ingredients! Their final identification was achieved using three main chemical tests:
Identification of unknown white solids (such as those found typical baking ingredients)
- pH testing to identify any bases or acids – for example baking soda is a base
- Biuret test to identify any protein based solids – for example
- Iodine test to identify any starch based solids – for example flour
- Silver nitrate test to identify any halide based solids – for example the sodium chloride in table salt, or potassium chloride in low-sodium salt alternatives
- Benedict’s test to identify any reducing sugars – for example sucrose
- Flame testing to identify the metal ions present – for example sodium in normal salt and potassium in “Lo-Salt”
Titration of sodium hydrogen carbonate (NaHCO3) in baking soda
The saboteur also tampered with the baking soda so that the other competitors’ cakes potentially would not rise properly or taste wrong. This was investigated using an acid-base titration technique to determine the amount of NaHCO3 present in the samples being tested
Thin-layer chromatography (TLC)
The saboteur also wrote a note (below) taunting the other competitors. By analysing the inks from a range of pens (one from each competitor) via a chromatogram (below right) and comparing these against the ink from the note, the saboteur can be caught and unmasked!

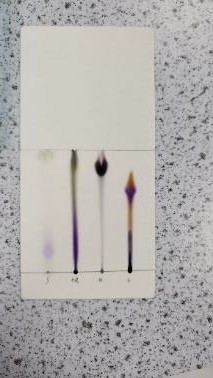
A short clip introducing how TLC works can be viewed *here*

Corey, Rania and Scarlett demonstrate the power of teamwork!

(L-R): Positive flame tests for potassium ions (purple flame) and sodium ions (yellow flame)
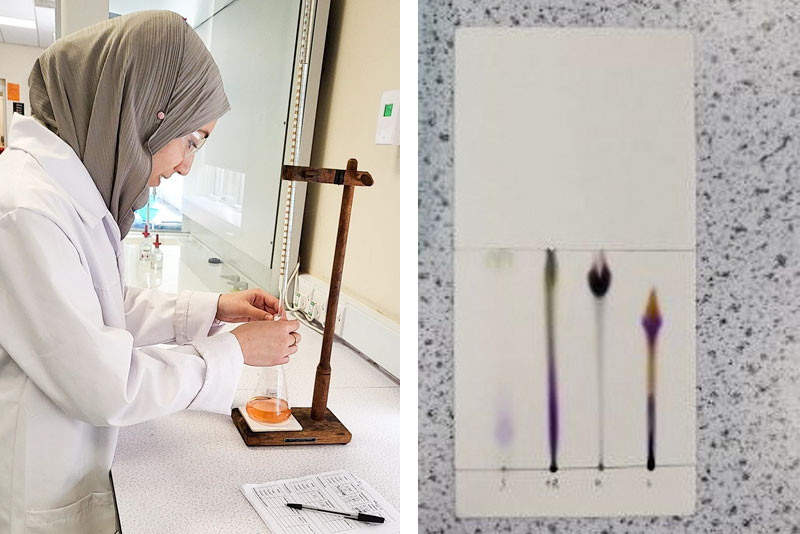
(L ) Rania carries out her titration! (R) Rania and Corey’s completed chromatogram

George and Angus carry out their white powder tests as outlined at the start of the article. Angus’s use of a top pan balance in the foreground is called gravimetric analysis whilst George setting up his titration in the background is known as volumetric analysis.

Achu, Nikah and Finley complete their solutions analysis of the white powders.

George imparts a little kinetic energy to his solution to assist with the dissolving process!

(L-R) Hector, Leah, Corey, Scarlett and Rania analyse their final results – a smile on the face of a research scientist always reveals quiet confidence in their findings!

A busy lab is a happy lab!

Emily and Anver are pleased with what their tests have revealed!

Leah completes her titration to determine the amount of sodium hydrogencarbonate in the baking soda.
