28th February 2023
Chemistry Society – extracting iron with a match head
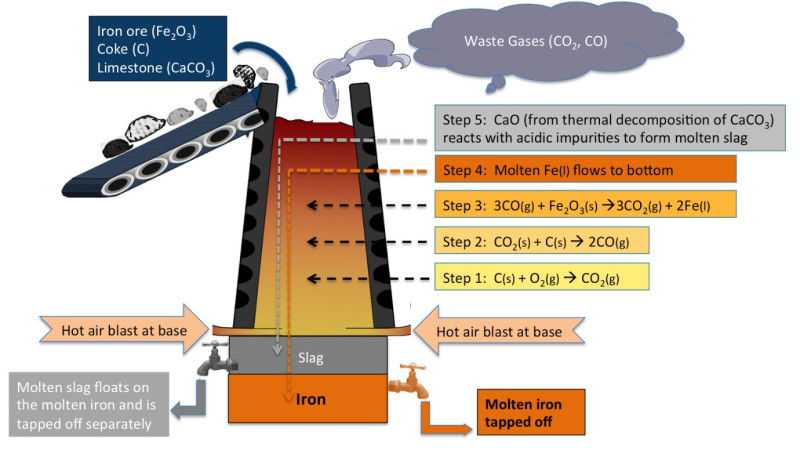
In chemistry society this month, we replicated the chemical reaction that takes place in a blast furnace when iron is extracted from its ore, haematite. The last blast furnace to operate in the UK was in Redcar near Middlesbrough but since the decline of the British steel industry in recent decades, it was recently demolished after many years of being slowly run down (more information on the BBC report here)
The chemical reaction itself which occurs is a redox reaction – redox was introduced in first-year A level chemistry at BHASVIC in November last year.
A video summarising these reactions outlined above, and introducing the practical we did can be found here. Some picture of our students carrying out the practical can be found below (scroll down)

Above: the tray of equipment each pair of students was provided with.

Above: Leah gets her Bunsen burner ready to light ahead of mixing all the reagents on the end of an unlit match. The match, once lit, will provide the activation energy but the reaction has been catalysed to allow it to operate at lower temperatures.

Above: George and Finn measure out their reagents and record the masses.

Above: Hector’s burning match now activates the reaction.

Above: Bea adds the sodium carbonate which catalyses the reaction at the lower temperature of a Bunsen burner compared to an actual blast furnace.

Above: George grinds the ash from the burnt match – which hopefully now contains some iron formed from the reduction of the iron (III) oxide!

Above: George tests out his ground up ash using a magnet – the iron (III) oxide is not magnetic so anything moving around over the magnet must be pure iron.

Above: Finn clarifies with me a possible way to extract more iron powder.
