06th December 2024
Chemistry Society
Investigating solid carbonates by thermal decomposition
To coincide with our teaching on Group 2 elements in first year chemistry, we investigated four “unknown” carbonate rocks in powdered form by way of thermal decomposition, according to this equation, where M is a Group 2 metal. A short video explaining the thermal decomposition process in depth can be found here.
One of the metal carbonates was ZnCO3 which although not a group 2 carbonate, happens to have the same ratio of metal ions to carbonate ions as regular group 2 elements, by way of Zn forming stable Zn2+ ions in the solid crystalline lattice of its carbonate.
MCO3(s) → MO(s) + CO2(g)
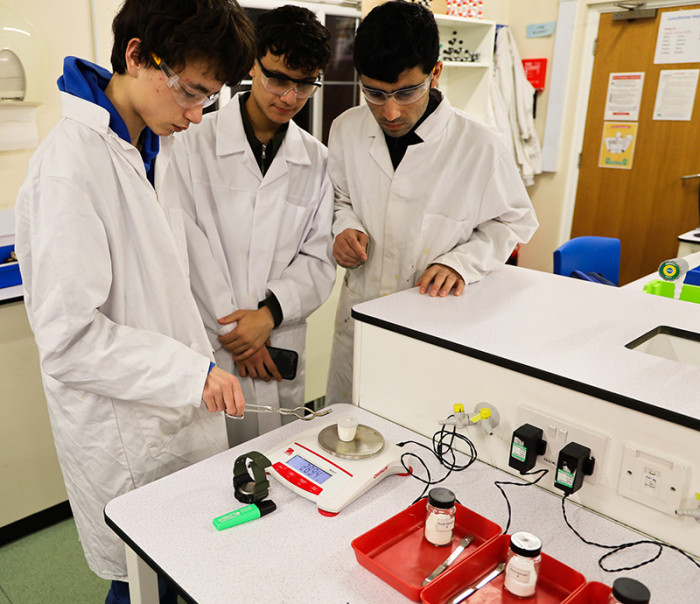
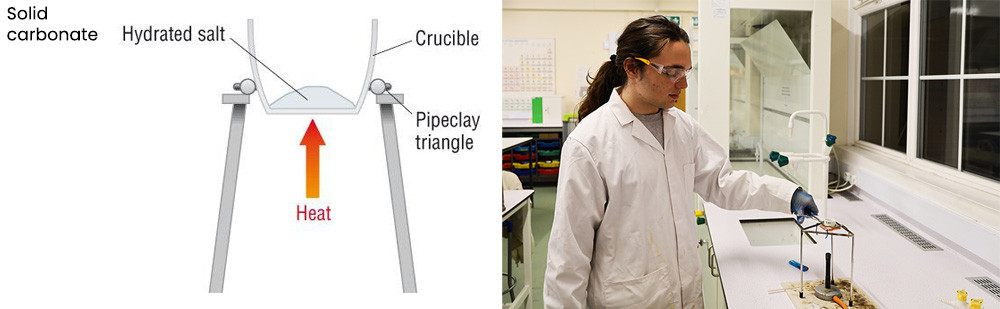
In a process of what is called “gravimetric analysis” we recorded the mass of MCO3 before heating and the mass of the oxide MO. We employed a technique called “heating to constant mass” in which continued heating and then weighing was carried out until two consecutive masses were the same – indicating all the carbon dioxide had been released and the reaction was complete. This is ably demonstrated below by A1 chemistry student Justin.
We also had some specially trained ESOL students joining us once again, and it was a pleasure to have their enthusiasm for learning and application of this to the techniques being used. On the next page is Mamadou’s group’s zinc oxide (this is the only one which turns yellow on heating) and their workings from their measurements.
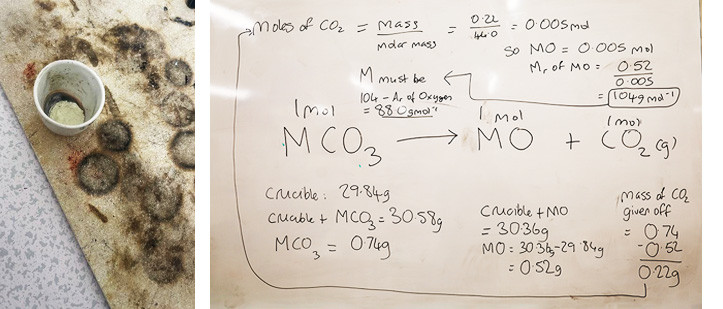
Above: yellow zinc oxide Above: Mamadou’s team’s measurements got close to zinc (88.0)
Below is the actual value for zinc (64.5 g mol-1). The “heating to constant mass” technique is quite hard to carry out accurately so well done for getting this close!

Below is a slide showing what we were expecting from each carbonate.
(the students had bottles A,B,C and D and that the four carbonates were one of the ones below).

Above: A1 chemistry student Noa (left) shows ESOL students Mohammed and Muhammad how to check whether the mass has stabilised and the reaction has finished.
Above: Mohammed and Muhammad take over the heating themselves whilst Noa watches on.