29th October 2018
Aspirin synthesis and analytical research
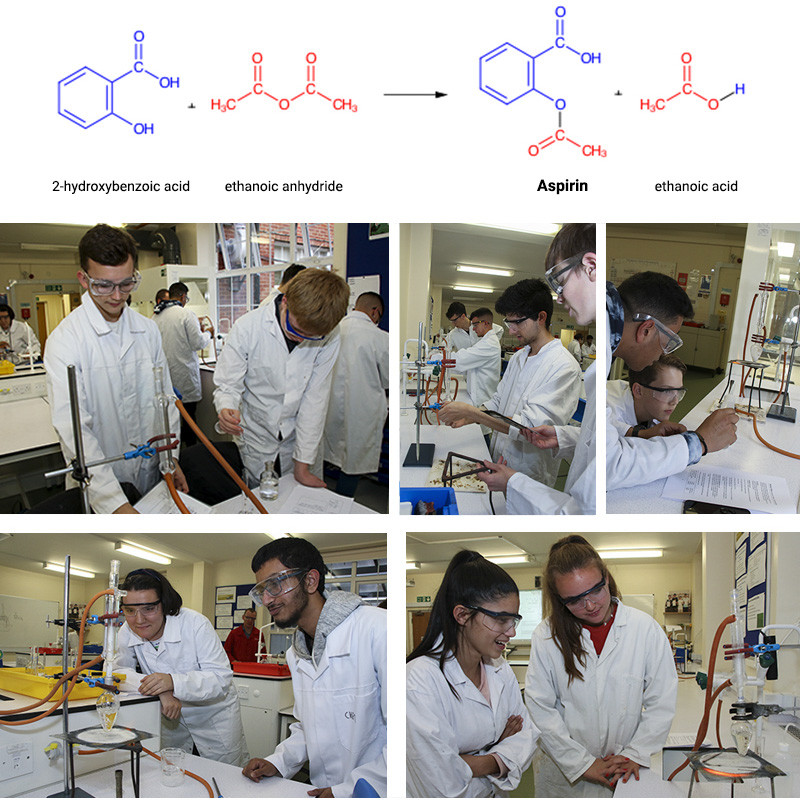
In the first half term, members of our Chemistry Society began the two-stage synthesis of the pharmaceutical substance aspirin (2-ethanoylyoxybenzoic acid) from the precursor ester molecule methyl-2-hydroxybenzoate, as we have been studying esters, carbonyl derivatives and chromatography in our second year lessons. In the photos below, students are hydrolysing the ester (methyl-2-hydroxybenzoate) using a strong alkali, sodium hydroxide. One of the products is 2-hydroxybenzoate which is reacted with a strong acid to make salicylic acid (2-hydroxybenzoic acid).
After filtration to purify and then leaving to dry, the next stage is to react this with ethanoic anhydride to make the aspirin product. After separation from the ethanoic acid side-product – the dried and purified sample will be sent off to RLC lab (www.rlclab.com ) for HPLC (high performance liquid chromatography) analysis. Our students will then be emailed the chromatograms for analysis. This is the second collaboration we have undertaken with RLC lab. Research such as this provides excellent experience and insight into the fields of pharmacy, medicinal chemistry and pharmacology.
Aspirin - Practical work preparatory activity
YouTube video: The aspirin screen experiment level 1
